Featured
Injection Ocrelizumab 1 Mg
The developmental and health benefits of breastfeeding should be considered along with the mothers clinical need for OCREVUS and any potential adverse effects on the. Ocrelizumab wird als Infusion über eine Vene intravenös verabreicht.
 Pml Reported In Ocrelizumab Treated Ms Patient Medpage Today
Pml Reported In Ocrelizumab Treated Ms Patient Medpage Today
Subscribe to Codify and get the code details in a flash.

Injection ocrelizumab 1 mg. Ocrevus 300 mg10 mL single-dose vial. OCREVUS ocrelizumab injection is a preservative-free sterile clear or slightly opalescent and colorless to pale brown solution supplied as a carton containing one 300 mg10 mL 30 mgmL single-dose vial NDC 50242-150- 01. South San Francisco CA.
Danach erfolgt alle sechs Monate eine Infusion von 600 mg Ocrelizumab. OCREVUS is a CD20-directed cytolytic antibody indicated for the treatment of patients with relapsing or primary. Les patients du Groupe B ont reçu linterféron bêta-1a Rebif 44 µg par injection sous-cutanée 3.
Ocrelizumab was excreted in the milk of ocrelizumab-treated monkeys. Lösungen von Ocrevus für die iv. All patients received standard of care mycophenolate mofetil or cyclophosphamide followed by azathioprine and were also permitted to receive IV or oral steroids.
1 mg 1 billable unit NDC. Injecting fuel IQ Our cylinder holds 474 mg of air. Ocrelizumab injection for intravenous use Initial US.
Ocrelizumab is given as an infusion into a vein. OCREVUS ocrelizumab Injection for intravenous infusion is a preservative-free sterile clear or slightly opalescent and colorless to pale brown solution supplied in single-dose vials. You may be given other medications to help prevent serious side effects of ocrelizumab.
This is referred to as 474 mgstroke. Injection ocrelizumab 1 mg Long Description. Each mL of solution contains 30 mg ocrelizumab glacial acetic acid 025 mg polysorbate 20 02 mg sodium acetate trihydrate 214 mg and trehalose dihydrate 40 mg at pH 53.
Ocrelizumab versus Placebo in Primary. Injection ocrelizumab 1 mg Code Added Date. C9494 is a valid 2021 HCPCS code for Injection ocrelizumab 1 mg or just Injection ocrelizumab for short used in Other medical items or services.
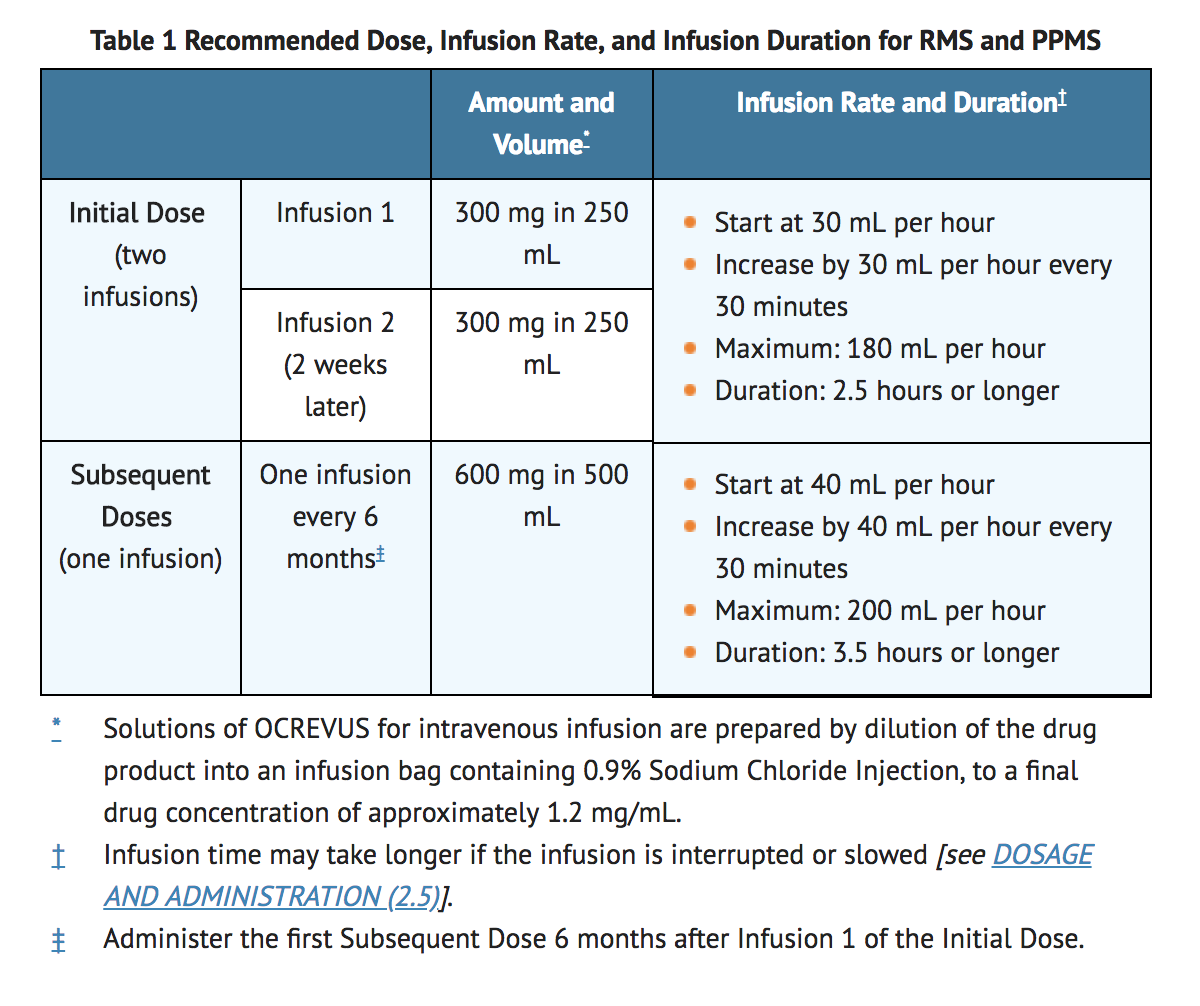
A healthcare provider will give you this injection. Es werden jeweils 300 mg im Abstand von 14 Tagen verabreicht. So every stroke of one piston will suck in 474 mg of air.
Ocrevus must be given slowly and the infusion can take from 25 to 35 hours to complete. Human IgG is excreted in human milk and the potential for absorption of ocrelizumab to lead to B-cell depletion in the infant is unknown. Receive placebo ocrelizumab 400 mg or ocrelizumab 1000 mg IV on Days 1 and 15 followed by a single infusion at Week 16 and every 16 weeks thereafter.
The following doses will be given once every 6 months. J2350 - Injection ocrelizumab 1 mg. Store OCREVUS vials at 2C to 8C 36F to 46F in the outer carton to protect from light.
HCPCS Code for Injection ocrelizumab 1 mg J2350 HCPCS code J2350 for Injection ocrelizumab 1 mg as maintained by CMS falls under Drugs Administered by Injection. So now we know how much air is in our cylinder 474 mg. Your first dose of ocrelizumab will be split into 2 separate infusions given 2 weeks apart.
HCPCS Code Details - C9494 HCPCS Level II Code. Infusion werden durch eine Verdünnung des Arzneimittels in einem Infusionsbeutel mit 09 igem Natriumchlorid auf eine Arzneimittel-Endkonzentration von ca. Injection ocrelizumab 1 mg Drugs administered other than oral method chemotherapy drugs J2350 is a valid 2021 HCPCS code for Injection ocrelizumab 1 mg used in Medical care.
You will be watched closely for at least 1 hour after receiving Ocrevus to make sure you do not have an allergic reaction to the medication. So our 474 cm 3 cylinder will hold 474 x 10 mg of air which is 474 mg of air. Drugs Administered Other Than Oral Method Chemotherapy Drugs Coding System.
Diesel burns at maximum efficiency at roughly 146 mg of air to 1 mg of fuel. A - Add procedure or modifier code Action Effective Date. Die erste Dosis Ocrelizumab 600 mg wird auf 2 Gaben verteilt.
We can inject some fuel. The primary endpoint was the ORR CRR and PRR at. Les patients traités par Ocrevus Groupe A ont reçu 600 mg tous les 6 mois Dose 1 en 2 perfusions intraveineuses de 300 mg administrées à 2 semaines dintervalle et les doses suivantes en une perfusion intraveineuse unique de 600 mg.
Montalban X Hauser SL Kappos L et al. Die erste Anschluss-Einzelinfusion sollte 6 Monate nach Infusion 1 der Anfangsdosis verabreicht werden.
 My Thrilling Journey What I Had To Do To Finally Get On A New Ms Medication By Kyle Reinhard Medium
My Thrilling Journey What I Had To Do To Finally Get On A New Ms Medication By Kyle Reinhard Medium
 Ocrevus Dosage Rx Info Uses Side Effects
Ocrevus Dosage Rx Info Uses Side Effects
 Ocrevus Fda Prescribing Information Side Effects And Uses
Ocrevus Fda Prescribing Information Side Effects And Uses
 Ocrelizumab Middel Tegen Pp Ms Msweb
Ocrelizumab Middel Tegen Pp Ms Msweb
 Ocrevus Ocrelizumab Injection For Intravenous Use South Delhi Pharma
Ocrevus Ocrelizumab Injection For Intravenous Use South Delhi Pharma
 300 Mg Ocrevus Ocrelizumab Injection Prescription Multiple Sclerosis Rs 28000 Box Id 23219938991
300 Mg Ocrevus Ocrelizumab Injection Prescription Multiple Sclerosis Rs 28000 Box Id 23219938991
Https Www Ocrevus Com Content Dam Gene Ocrevus Hcp Pdfs Ocrevus Multiple Sclerosis Dosing And Administration Guide Pdf
Ocrevus Ocrelizumab Ingrid Schrijft
 Ocrevus Inj 300mg 10ml Vial 1 S Price In Pakistan
Ocrevus Inj 300mg 10ml Vial 1 S Price In Pakistan
 Buy Ocrevus Ocrelizumab Price Costs Thesocialmedwork
Buy Ocrevus Ocrelizumab Price Costs Thesocialmedwork
 Ocrevus Ocrelizumab Injection Uses Dose Side Effects Ms
Ocrevus Ocrelizumab Injection Uses Dose Side Effects Ms
 Injection Ocrelizumab 300 Mg For Hospital 1 Vial Rs 10 Bottle Id 23367254255
Injection Ocrelizumab 300 Mg For Hospital 1 Vial Rs 10 Bottle Id 23367254255

Comments
Post a Comment