Featured
Avise Sle Monitor Test
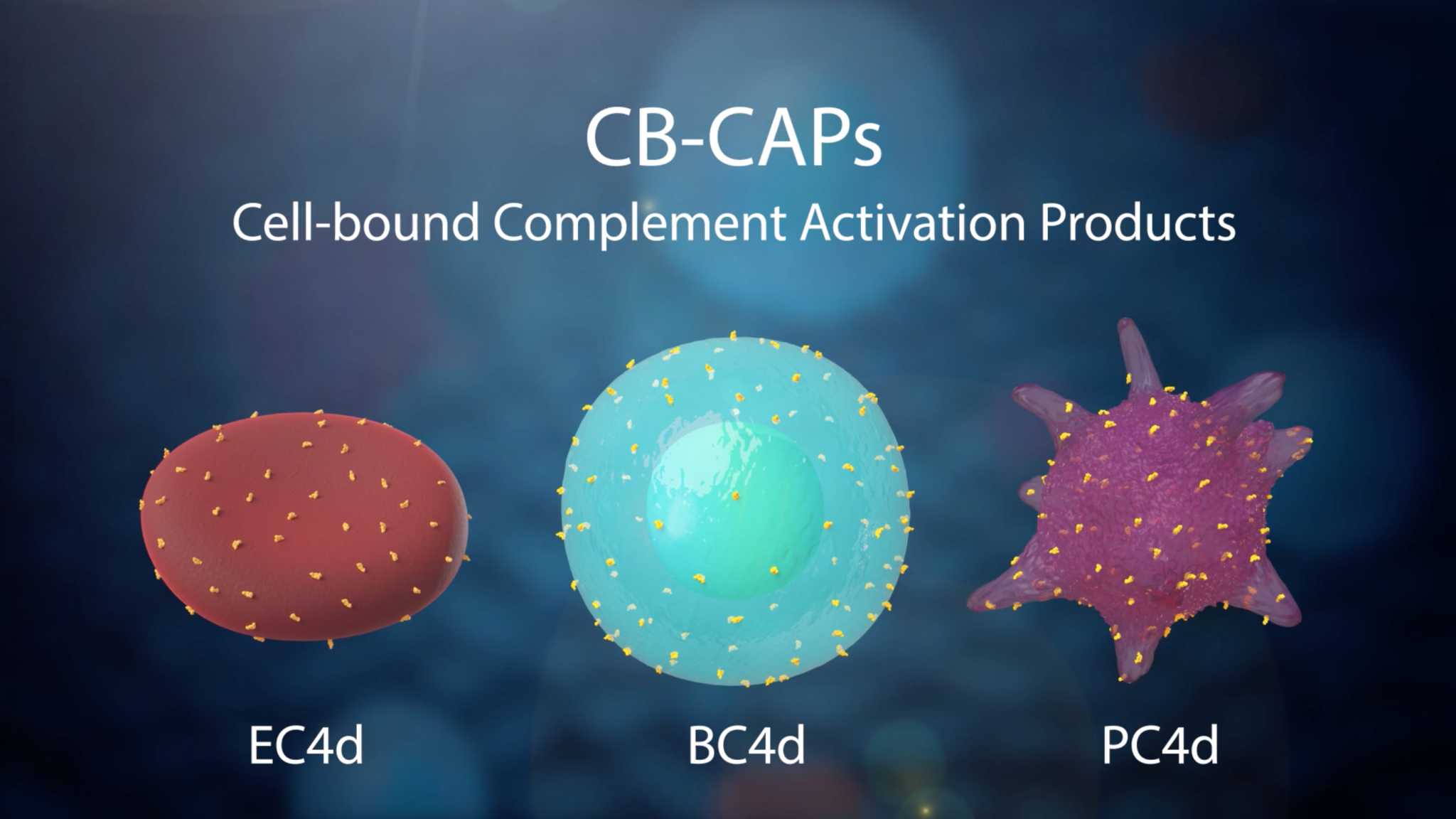
A 10-marker diagnostic test containing CB-CAPs and SLE associated markers designed to aid healthcare providers in a timely differential diagnosis of SLE. Background The AVISE Connective Tissue Disease CTD test uses autoantibody erythrocyte-bound C4d EC4d and B-cell-bound C4d BC4d levels to aid in diagnoses of SLE other CTDs and fibromyalgia.
 Advanced Lupus Test Lupus Diagnosis Test Exagen Inc
Advanced Lupus Test Lupus Diagnosis Test Exagen Inc
The AVISE SLE Monitor test can help you and your doctor regularly track your diseases activity level making you aware of abnormalities possibly before symptoms begin to show up and helping you prevent damage to your body.

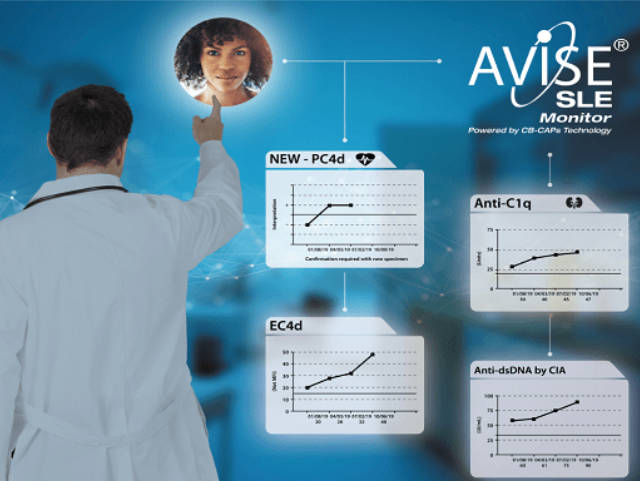
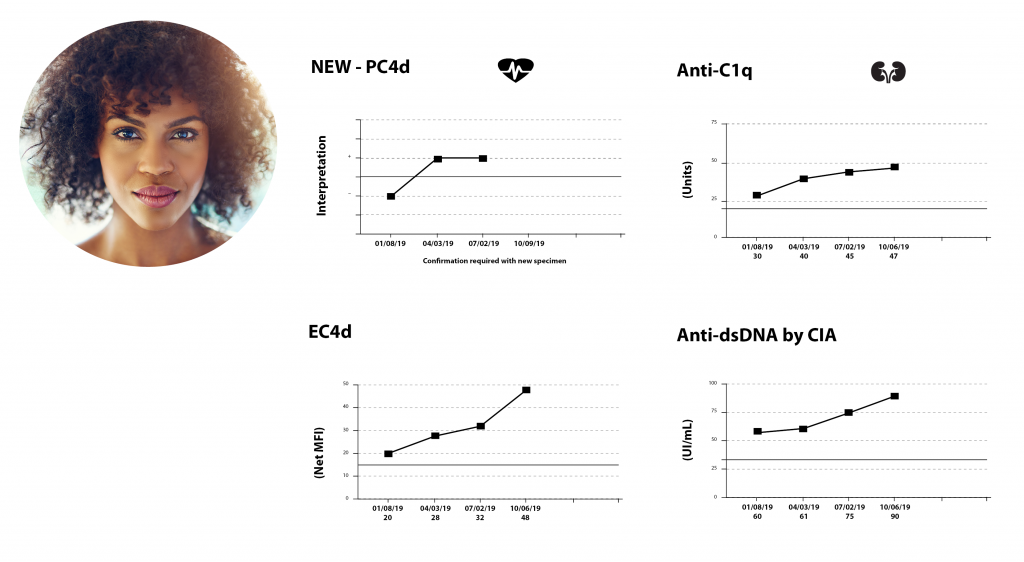
Avise sle monitor test. AVISE SLE Monitor AVISE SLE Monitor is an advanced bloodtest that provides important data to assist physicians anytime they assess the condition of a patient with SLE. AVISE SLE Monitor is composed of the following biomarkers Biomarkers associated with SLE disease activity Erythrocyte-bound C4d - EC4d. Specialized test to aid in the diagnosis and management of patients with antiphospholipid syndrome APS.
The test includes the following assays. AVISE SLE Monitor Test Report. AVISE Lupus contains the following biomarkers.
AVISE SLE Prognostic AVISE SLE Monitor AVISE Test Components and Descriptions PC4d C3 C4 RA RF IgM RF IgA Thyroid TPO TG PSPT IgG. Like CRP the ESR is not specific to lupus. This monitoring test can help your physician determine what is happening below the surface and may help you detect flares or avoid a flare altogether.
The test may be useful for people who have a positive antinuclear antibodies ANA test or anyone concerned they may have lupus or a similar autoimmune condition. The AVISE SLE Monitor test should be considered any time the condition of a SLE patient is being assessed. AVISE SLE Monitor is an advanced SLE monitoring test comprised of six specialized biomarkers including patented EC4d and PC4d to help assess patients with SLE.
AVISE CTD is an advanced autoimmune rheumatic disease test specifically designed to aid physicians in the differential diagnosis of systemic lupus erythematosus SLE. We evaluated the utility of the AVISE CTD test in predicting SLE disease development and damage progression. With SLE Monitor Order Type Tube Requirements Up to 2 AVISE tests 3 or more AVISE tests one- 10 mL whole blood EDTA lavender tube one- 5 mL serum SST tiger top tube two- 10 mL whole blood EDTA lavender tubes one- 5 mL serum SST tiger top tube AVISE Specimen Requirements AVISE Specimen Submission PREPARE SPECIMEN COLLECTION KIT FOR.
AVISE SLE Prognostic The Specialized SLE Prognostic Test for Rheumatologists AVISE SLE Prognostic is a 10-marker panel developed to help assess a patients potential risk for Thrombosis Cardiovascular events Lupus Nephritis and Neuropsychiatric Lupus. AVISE Lupus is a 10-marker diagnostic test containing Cell-Bound Complement Activation Products CB-CAPs and SLE associated markers designed to aid healthcare providers in a timely differential diagnosis of SLE. AVISE CTD is the only diagnostic test powered by patented Cell-Bound Complement Activation Products CB-CAPs stable biomarkers of complement activation to help physicians better assess patients with suspected SLE.
Methods Patients who had undergone AVISE CTD testing were assessed for SLE. AVISE CTD is a blood test that can help doctors diagnose lupus and other autoimmune diseases like rheumatoid arthritis Sjögrens syndrome or scleroderma. AVISE SLE Monitor.
This test employs unique stateof--the-art biomarkers with a demonstrated significant correlation with clinical improvements in SLE. AVISE SLE Prognostic Developed as an add-on test to AVISE CTD AVISE Prognostic helps assess a patients potential risk for organ involvement through Thrombosis Cardiovascular events Lupus Nephritis and Neuropsychiatric Lupus. The SLE lupus test solution from.
If the patient is also being monitored with AVISE HCQ the AVISE SLE Monitor test report will also include an AVISE HCQ test result page. Limitations of the test. Our AVISE SLE Systemic Lupus Erythematosus test incorporates a powerful combination of biomarkers for accurate results.
The Test Includes the Following Assays Anti-C1q Anti-Phosphatidylserine Prothrombin PSPT IgM IgG. LM1031 320 Advance Patient Notification APN Do not use for MedicareMedicaidTricare Patients Please submit thi s form with your AVISE test requisition. AVISE SLE Prognostic is a 10-marker panel developed to help assess a patients potential risk for Thrombosis Cardiovascular events Lupus Nephritis and Neuropsychiatric Lupus.
The AVISE SLE Monitor test created by Exagen tests for specialized lupus biomarkers including those that are not found in traditional blood tests. This test could be used to monitor inflammation which could indicate changes in disease activity or response to treatment.
 Advanced Diagnostic Test For Systemic Lupus Erythematosus
Advanced Diagnostic Test For Systemic Lupus Erythematosus
 Systemic Lupus Erythematosus Sle Test Exagen
Systemic Lupus Erythematosus Sle Test Exagen
 Systemic Lupus Erythematosus Sle Test Exagen
Systemic Lupus Erythematosus Sle Test Exagen
 Systemic Lupus Erythematosus Sle Test Exagen
Systemic Lupus Erythematosus Sle Test Exagen
 Systemic Lupus Erythematosus Sle Test Exagen
Systemic Lupus Erythematosus Sle Test Exagen
 Avise Testing Supplies Order Form
Avise Testing Supplies Order Form
 Sle Test Systemic Lupus Erythematosus Test Avise Testing
Sle Test Systemic Lupus Erythematosus Test Avise Testing
 The 2 Tiered Model Of The Avise Lupus Test Download Scientific Diagram
The 2 Tiered Model Of The Avise Lupus Test Download Scientific Diagram
 Exagen Launch Of Novel Biomarker Pc4d Associated With Thrombosis In Lupus Exagen Inc
Exagen Launch Of Novel Biomarker Pc4d Associated With Thrombosis In Lupus Exagen Inc
 Advanced Lupus Test Lupus Diagnosis Test Exagen Inc
Advanced Lupus Test Lupus Diagnosis Test Exagen Inc
 Lupus Diagnosis And Management 3 Tests That May Be Right For You Lupus Chick
Lupus Diagnosis And Management 3 Tests That May Be Right For You Lupus Chick



Comments
Post a Comment